Research
The Texas Heart Institute is recognized internationally for research programs in cardiology, cardiovascular surgery, regenerative medicine and pathology. Our physicians and scientists conduct research ranging from gene therapy, stem cells and regenerative medicine, to ventricular assist devices (VADs) and artificial hearts.
Clinical Trials
Researchers at The Texas Heart Institute conduct clinical trial studies to test the safety and efficacy of potential new devices, techniques and therapies to treat patients suffering from various cardiovascular issues. Learn more about what clinical trials are and the current studies our researchers are conducting.
Electrophysiology Clinical Research & Innovations
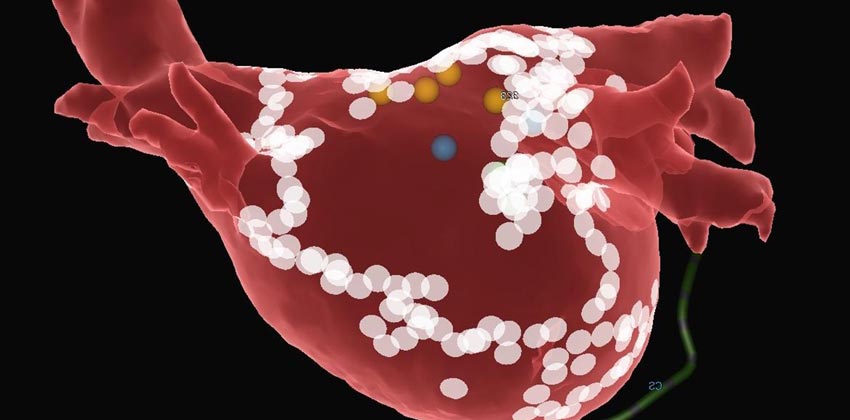
Since its founding by Dr. Denton A. Cooley in 1962, The Texas Heart Institute has been an internationally recognized leader in surgical innovation and developed state of the art infrastructure for translational and clinical cardiac arrhythmia research and innovation to carry on the tradition of its founder. The Texas Heart Institute’s Electrophysiology Clinical Research & Innovations department (ECRI) is pioneering some of the most ground breaking cardiac arrhythmias research and management today in collaboration with institutions across the US.
Center for Clinical Research

Through our clinical research we are gaining answers to important questions about the prevention, diagnosis and treatment of medical conditions.
Center for Preclinical Surgical & Interventional Research
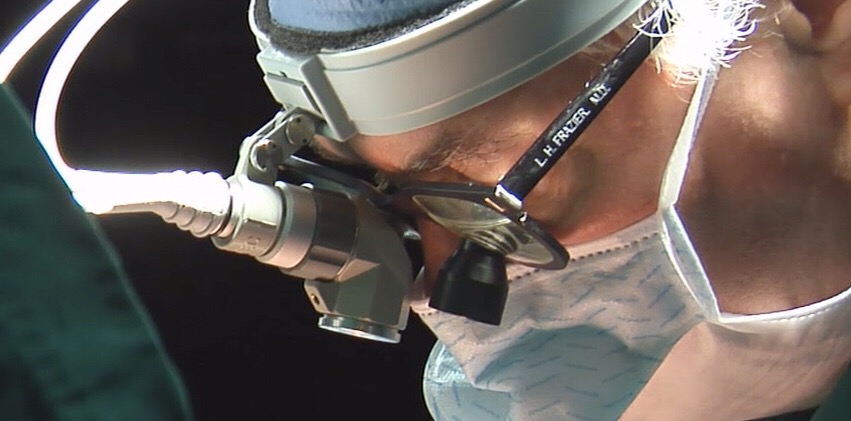
One of our longest-standing research departments, the Cardiovascular Surgery Research Laboratories seek to advance medical knowledge and develop new ways to repair and replace diseased organs to enhance and extend life. Researchers in this department have been involved in the development of nearly every mechanical circulatory support device in use today.
Center for Women’s Heart & Vascular Health

The purpose of the Center for Women’s Heart & Vascular Health is to strengthen the Institute’s focus on women-oriented research, address the significant gender gaps that exist in cardiovascular medicine by educating women and physicians caring for women, and investigate the causes of excess cardiac risk in minority populations.
Cardiomyocyte Renewal Laboratory
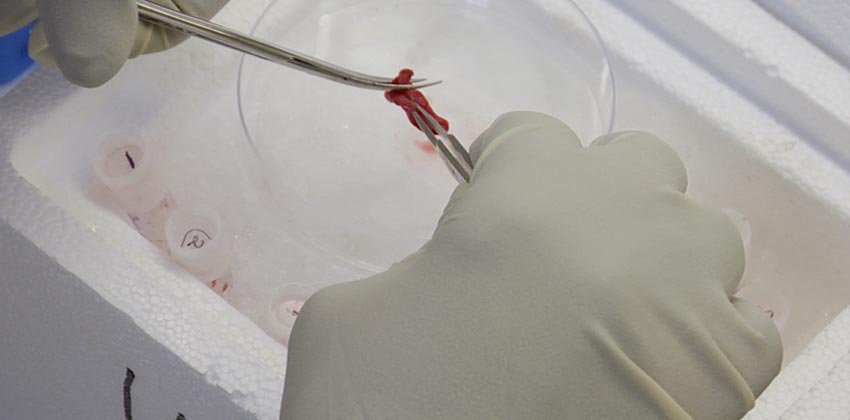
The Cardiomyocyte Renewal Laboratory focuses on understanding how specialized signaling pathways are connected to adult tissue development and regeneration. The team’s research studies are designed to decipher the molecular pathways that are active in cardiac disease states and develop therapies to control them to promote heart muscle regeneration.
Cardiovascular Pathology
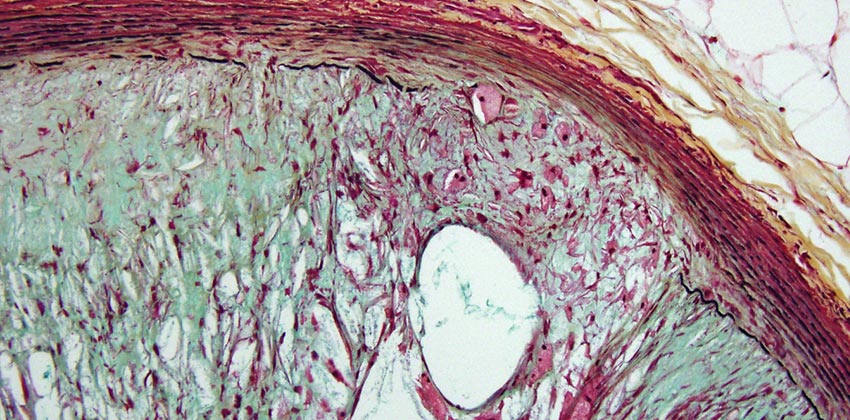
The Cardiovascular Pathology Research department provides specialized pathology and histology services to THI research investigators and to external researchers and organizations on a fee-for-service basis. Additionally, the team conducts original research related to various areas of cardiology.
Regenerative Medicine Research
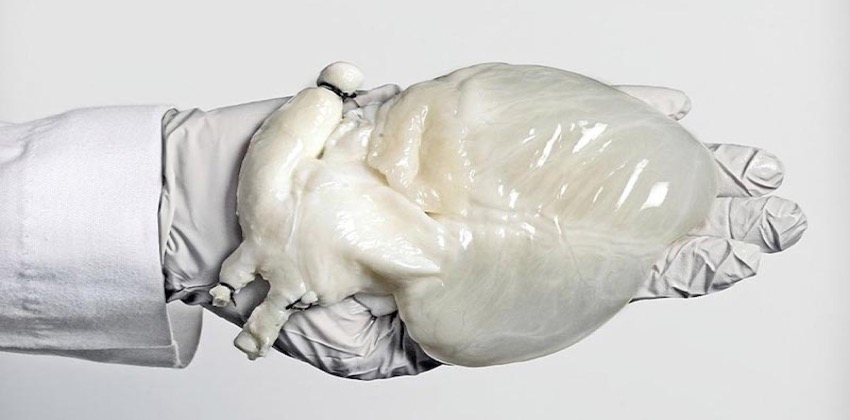
Our Regenerative Medicine Research department is dedicated to unveiling the next discovery in this promising, emerging field. Primarily, the team is focused on furthering the science of engineering bio-artificial organs and tissues, understanding aging as a failure of stem cells, and identifying sex differences in cardiovascular disease and regenerative medicine treatments.
Center for Heart Valve Disease
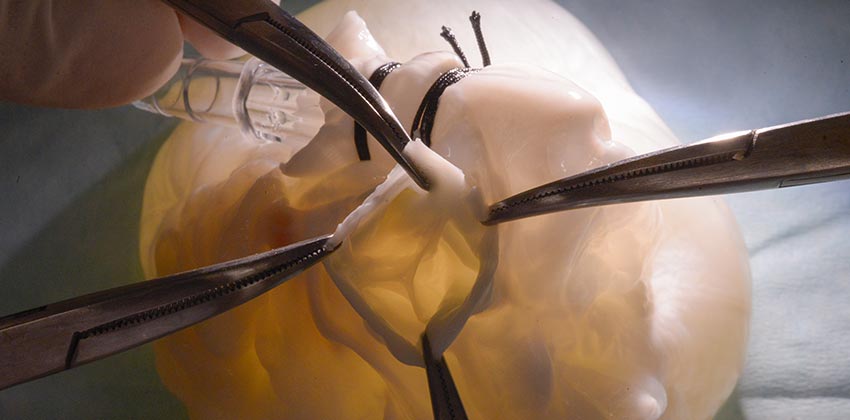
With instances of valvular heart disease on the rise, finding new and innovative ways to diagnose and treat these conditions is critical. The Center for Heart Valve Disease focuses on all aspects of valvular heart disease, including improved replacement procedures with less invasive technology.
Electrophysiology Basic Research

The Electrophysiology Basic Research lab aims to gain critical insights about heart rhythm abnormalities and the electrophysiological mechanisms that cause them. By furthering our understanding of the basic science related to arrhythmias, researchers will be able to improve techniques to detect and treat them.
Molecular Cardiology Research
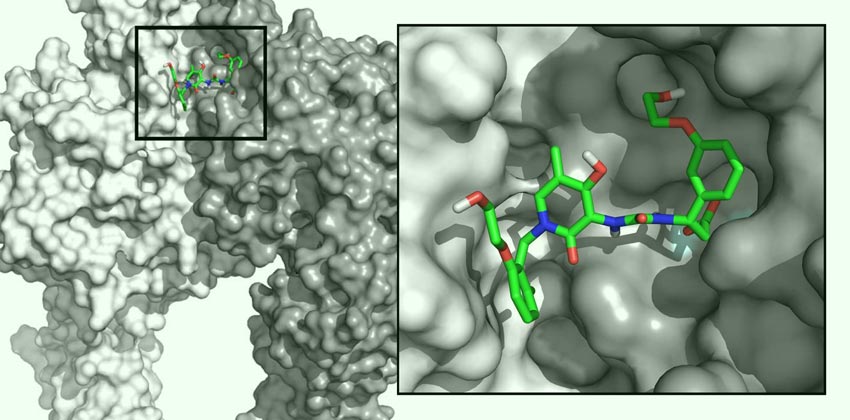
The Molecular Cardiology Research Laboratories seek to better understand the molecular mechanisms of heart disease while pushing boundaries in the research and development of small molecule and cell-based therapeutics. The team leverages their expertise in molecular biology to discover ways to improve the treatment of various cardiovascular diseases.
Innovative Device & Engineering Applications (IDEA) Lab

The Innovative Device & Engineering Applications (IDEA) lab is focused on developing and evaluating cutting-edge cardiovascular medical devices. The lab specializes in minimally invasive left ventricular assist devices (LVADs) and various cardiovascular testing/training models for mechanical circulatory systems and interventional medical devices.
Stem Cell Center

With initiatives focused in basic and clinical research, the Stem Cell Center seeks to leverage the regenerative properties of stem cells to help patients suffering from cardiovascular disease by improving treatment options and even reversing the effects of disease or damage to the heart. The team also utilizes their discoveries to further stem cell therapies across all areas of medicine.




