The Molecular Cardiology Research Laboratory (MCRL), under the leadership of Richard A. F. Dixon, Ph.D., leverages its unique expertise in small molecule therapeutics and molecular biology to treat, diagnose, and prevent cardiovascular disease. Many members of the team have pharmaceutical industry experience with track records of successful drug discovery and development. Two lead investigators in the department have been elected to the National Academy of Inventors within the last year. In keeping with Dr. Willerson’s storied history of translational research, the team has used small molecule drugs, gene therapy, and stem cell technologies to target an assortment of cardiovascular diseases. A milestone was achieved this year when a small molecule drug discovered and developed in the MCRL here at THI entered human clinical testing in October.
Texas Heart Institute’s molecular discovery at the heart of a new vaccine development program
Texas Heart Institute Molecular Cardiology Research Scientist Elected Senior Member of the National Academy of Inventors
Visionary THI Scientist Honored by the National Academies of Inventors
Atherosclerosis and Imaging of Vulnerable Plaque
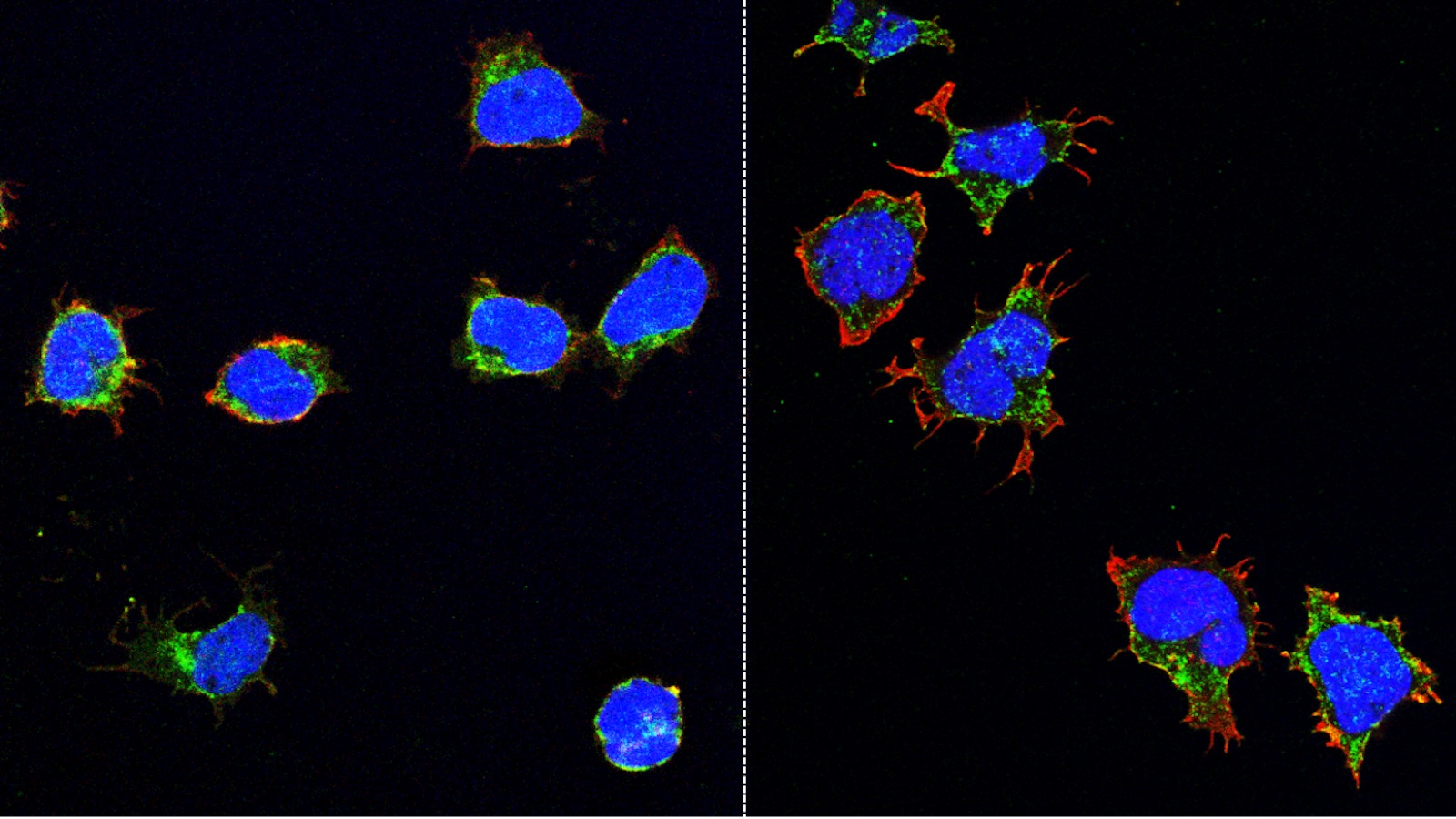
Atherosclerosis is an inflammatory disease. As such, inflammatory cells account for a high percentage of the cells that make up an atherosclerotic plaque. Indeed, the initial migration of inflammatory cells into tissue and their subsequent conversion to foam cells is considered an initial trigger of plaque development. MCRL scientists have developed small molecule drugs that can prevent inflammatory cells from entering atherosclerotic plaque. In animal models of atherosclerosis, drug treatment reduced the inflammatory plaque burden. As an extension of this work and in collaboration with investigators at Texas Children’s Hospital, MCRL scientists have modified these drugs to serve as targeting agents that selectively deliver magnetic resonance imaging (MRI) modalities to inflammatory cells in atherosclerotic plaque. This would allow for the use of a non-invasive imaging modality to detect plaques in patients at an early stage of development, thereby allowing for proactive intervention with preventative treatments. The imaging agent can also be modified to deliver drugs to potentially reduce plaque burden and therefore function as both a diagnostic and targeted therapeutic. By interfering with the trafficking of inflammatory cells, these small molecule drugs and/or targeting agents potentially could be used to treat or diagnose additional diseases, including hematologic cancers and autoimmune diseases such as multiple sclerosis and inflammatory bowel disease.
Cell adhesion research could lead to a new pain alternative for patients with inflammatory conditions
Peripheral Arterial Disease
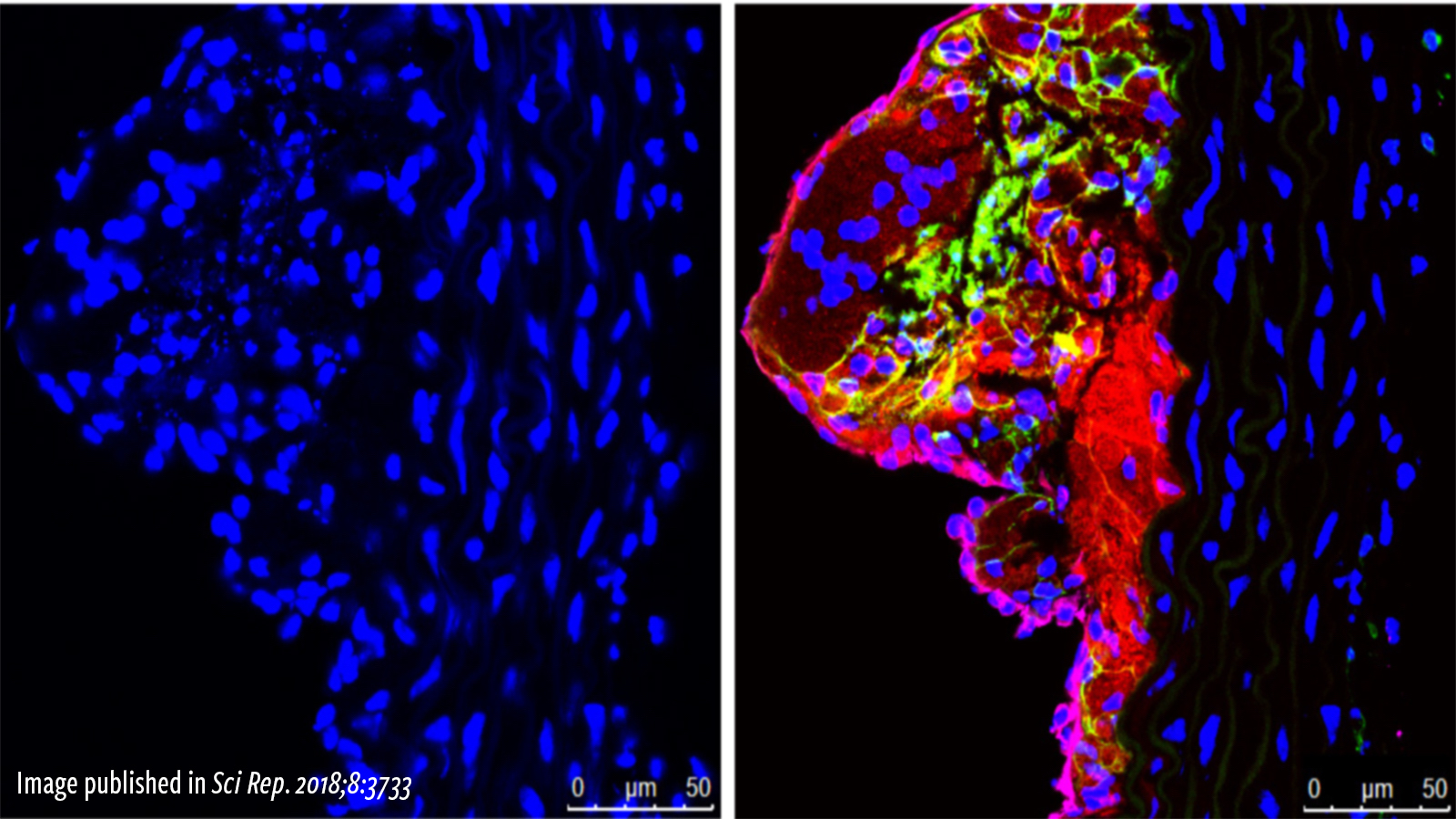
Similar to coronary artery disease in the heart, atherosclerotic plaque can lead to peripheral arterial disease (PAD), which is characterized by reduced blood flow to the limbs, typically the legs. The damage to the skeletal muscles in PAD leads to pain and cramping in the legs and, if left untreated, possibly to gangrene and amputation. Despite the high incidence of PAD, current therapies are ineffective in replacing damaged tissue with new functional muscle tissue. Therefore, identifying new treatment approaches for PAD is one of the main missions of the American Heart Association. Because repair of damaged skeletal muscles requires muscle generation from stem cells, the MCRL has developed novel gene-therapy type approaches based on the seminal discoveries of Dr. James Martin, Director of the Cardiomyocyte Renewal Laboratory at THI. In animal models of PAD, treatment of injured skeletal muscle with such therapies results in activation of the muscle stem cells and dissemination of angiogenic signals to the associated blood vessels, leading to new blood vessel formation and skeletal muscle regeneration.
Biosystems Modeling for Personalized Medicine
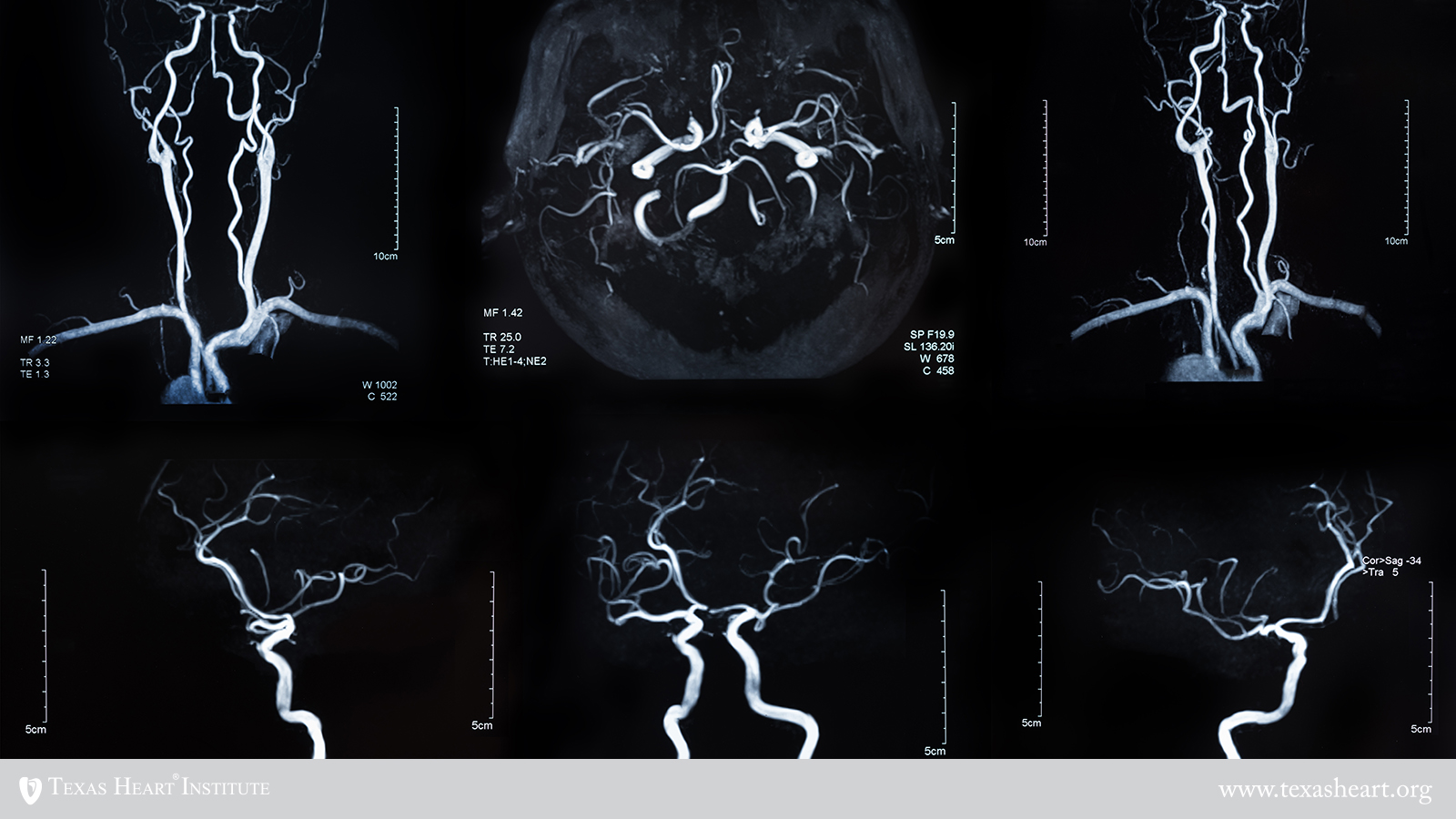
MCRL investigators have developed computer-based modeling systems to analyze the architecture of blood vessels that supply the heart and the brain. These systems can be used to create new strategies for personalized patient risk assessment and for helping to identify which therapies to use for better patient care. In a joint initiative between THI and UT Austin’s James T. Willerson Center for Cardiovascular Modeling and Simulation, investigators are using computational modeling toolsets to personalize and optimize agents that image and treat vulnerable plaque such as those described above. In conjunction with patient-specific attributes to maximize targeting efficiency, the models will ultimately enable physicians to tailor treatment for every patient and predict with a high degree of accuracy its effects on the patient before administering it. In collaboration with researchers at Texas Children’s Hospital and UT Health Science Center, the group has also developed an image-based computational modeling toolset to noninvasively assess stroke risk in patients with cerebrovascular disease. The toolset, developed with support from the National Institutes of Health (NIH), can be used to help ensure that patients at risk of recurrent stroke are properly followed and treated before strokes occur
Texas Heart Institute research scientist receives grant to help kids at risk for Moyamoya Disease
 Vaccine Development
Vaccine Development
Physician researchers at THI and elsewhere have established a link between respiratory infections, especially influenza, and acute myocardial infarction. Although vaccines are available to prevent the flu, the response rate among those over the age of 65 is greatly reduced due to an underactive immune system. A team of MCRL scientists has developed a small molecule drug that can “ramp up” the immune response. In animal models, the drug enhances the response to several vaccines including influenza. This first-in-class drug has been out-licensed to a small pharmaceutical company that has since initiated an NIH-funded phase I clinical trial in healthy volunteers to determine its safety and optimum dosing regimens. This would set the stage for future clinical trials in the setting of geriatric influenza. In the weeks after influenza infection, patients are at an increased risk of heart attack. Improving the efficacy of influenza vaccinations in the elderly could help reduce this risk. Based on its unique mechanism of action on the immune system, the drug has also been tested in animal models of cancer in collaboration with MD Anderson Cancer Center; these studies have shown that the drug can enhance the response of checkpoint blockade antibodies against solid tumors such as melanoma.
Technology Co-Invented by THI Scientists In the News
Texas Heart Institute Discovery to Enhance the Immune System Moves Closer to Clinical Testing
Stem Cell Transplant
Umbilical cord blood has become the preferred source of stem cells for patients in need of a bone marrow transplant because a less restrictive donor match is required, and cells can be readily stored for future use. One drawback, however, is that there are significantly fewer stem cells in cord blood preparations as compared to other sources, which restricts their use primarily to pediatric populations. Fewer stem cells also mean it takes longer for the immune system to reconstitute itself in the recipient, resulting in a higher incidence of opportunistic infections and graft failures. Finding a means to enhance stem cell engraftment and accelerate the time to immune reconstitution is a primary goal in the transplant field. The MCRL team has developed a small molecule drug that can enhance the binding of cord blood stem cells to the bone marrow microenvironment. In animal model studies of cord blood transplant funded by the NIH, the drug enhanced engraftment of cord blood stem cells into the bone marrow and increased the rate of immune reconstitution.