Expanding Hippo Pathway Studies From the Heart to the Head: A Novel Role of Hippo Pathway Proteins in Craniofacial Bone Formation
The essential Hippo pathway that provides “stop” signals for organ and tissue growth has been explored extensively at The Texas Heart Institute (THI) by researchers seeking new therapies for heart repair and regeneration. A newly published, collaborative study by investigators at The University of Texas Health Science Center at Houston (UTHealth), THI, and other institutions reveals that Hippo pathway proteins are also critically involved in the development of bones in the skull and face (craniofacial bones).
In the THI Cardiomyocyte Renewal Laboratory directed by James F. Martin, MD, PhD, the past decade has seen a number of seminal discoveries involving the Hippo signaling pathway. After birth, artificially manipulating the Hippo pathway to send “start” signals for cell division can promote tissue regeneration. However, when the Hippo pathway is not properly regulated, it can also lead to disease development. This landmark research by Dr. Martin’s group has been important in moving the field of developmental and regenerative biology forward.
Although many of the Cardiomyocyte Renewal Laboratory’s studies of the Hippo pathway have involved the heart, Jun Wang, PhD, who trained at Baylor College of Medicine as a graduate student and postdoctoral fellow under the direction of Dr. Martin, has expanded the investigation of Hippo pathway function to the development of craniofacial bones. In addition to holding an adjunct THI Professional Staff membership, Dr. Wang is now a tenured Associate Professor in the Department of Pediatrics at the UTHealth Houston McGovern Medical School. In collaboration with multiple research teams including THI, Dr. Wang has been working to understand the molecular and genetic regulation of the neural crest. The neural crest is a temporary structure that forms during the early stages of fetal development and is composed of multipotent cells that can grow into multiple cell types and contribute to various tissues including cranial bone and heart. Her team’s ground-breaking findings have revealed that two proteins involved in the Hippo pathway—called Yap and Taz—play a critical role in promoting neural crest cells to undergo osteogenesis (bone formation). Their findings have been recently published in the American Association for the Advancement of Science journal Science Signaling. The study is also featured on the cover of the journal.
Specifically, Dr. Wang and her collaborators have found that Hippo pathway proteins Yap and Taz help guide the fate of neural crest cells by promoting their specialization into bone cells of the skull and face while preventing them from becoming misplaced cartilage. Most of the craniofacial skeleton is derived from neural crest cells. Importantly, abnormal neural crest development gives rise to various common congenital human birth defects such as cleft lip/palate, malformations in the heart, and genetic syndromes. Therefore, understanding the mechanisms of neural crest development is of great clinical value because it may lead to new options for treating birth defects.
In their recently published study, Dr. Wang and researchers used a step-by-step experimental approach that ranged from studies in cultured neural crest cells to genetically modified mice to show that Yap and Taz promote neural crest cells to undergo osteogenesis while preventing them from undergoing chondrogenesis (cartilage formation). As Dr. Wang explains, “when Yap and Taz are active, they bind to other proteins to coordinately regulate the expression of specific genes. Therefore, we knew we could learn a lot about their role in determining cell fate by comparing the types of genes that were expressed in neural crest cells in the presence or absence of Yap and Taz.”
As such, they found that Yap and Taz activate the expression of bone-forming genes in neural crest cells while preventing the expression of cartilage-forming genes. Importantly, they found mice that lacked the Yap and Taz proteins had defective facial and skull bone formation and abnormal cartilage formation when compared with control mice that had Yap and Taz, supporting that Yap and Taz are important for craniofacial bone formation.
The researchers also uncovered some important details for how Yap and Taz promote cranial bone formation. For example, they found that Yap and Taz have overlapping functions. In addition, they found that Yap and Taz promote the formation of cranial bone partly through interactions with another important signaling pathway (the Wnt–β-catenin pathway).
In Dr. Wang’s words, “This study has helped to answer the fundamental biologic question of how the cell fate of multipotent neural crest cells is determined. We now know how Hippo signaling is involved in fine-tuning the intricate process that forms the craniofacial skeleton.” As these researchers continue to explore more about how Yap and Taz function in neural crest cell fate determination, these findings promise to open new avenues of research. “Our study has broad implications that may extend to other types of stem cells other than neural crest cells. We may have just touched the tip of the iceberg,” concludes Dr. Wang.
Read Report
Zhao X, Tang L, Le TP, Nguyen BH, Chen W, Zheng M, Yamaguchi H, Dawson B, You S, Martinez-Traverso IM, Erhardt S, Wang J, Li M, Martin JF, Lee BH, Komatsu Y, Wang J. Yap and Taz promote osteogenesis and prevent chondrogenesis in neural crest cells in vitro and in vivo. Sci Signal. 2022 Oct 25;15(757):eabn9009. doi: 10.1126/scisignal.abn9009.
Figure Details
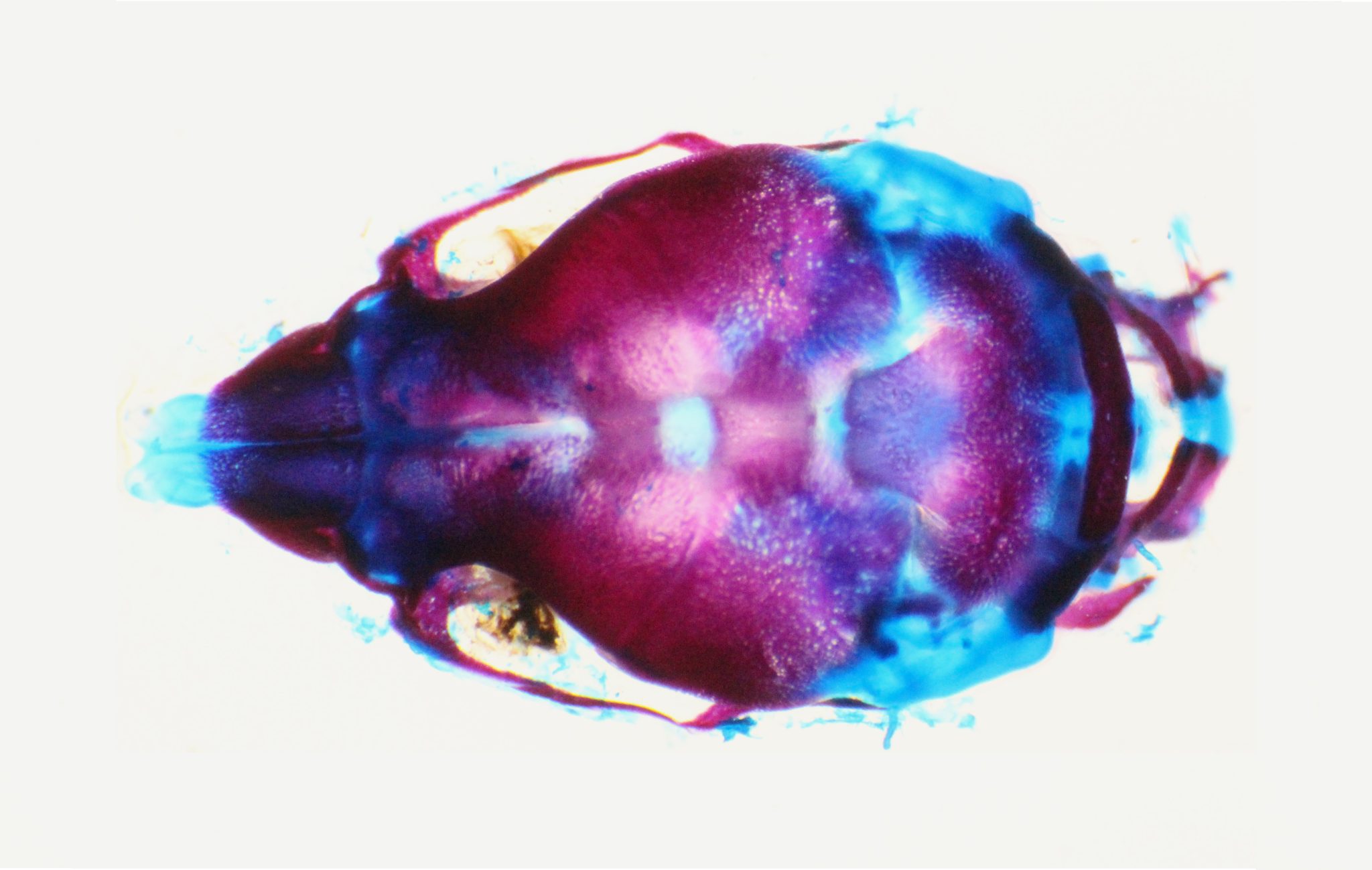
Image of a skull from a normal control mouse on postnatal day 1 stained to show bone in red and cartilage in blue. Image courtesy of Dr. Xiaolei Zhao, Department of Pediatrics, McGovern Medical School, University of Texas Health Science Center at Houston.
News Story By Nicole Stancel, PhD, ELS(D)




