Researchers at The Texas Heart Institute Pioneer Light-Responsive and Wireless Cardiac Stimulation Technologies

Researchers at The Texas Heart Institute (THI)—Camila Hochman-Mendez, Fernanda C. Paccola Mesquita, Ernesto Curty da Costa, Angel Moctezuma-Ramirez, and Abdelmotagaly Elgalad—have co-authored two groundbreaking studies exploring a revolutionary bioprinted cardiac tissue platform. The papers highlight the development of optoelectronically active bioprinted scaffolds, which integrate light-responsive materials to create electrically generative, wireless tissue constructs for cardiac therapy.
Ultrathin Rubbery Bio-Optoelectronic Stimulators
The first study, “Ultrathin Rubbery Bio-Optoelectronic Stimulators for Untethered Cardiac Stimulation”, introduces the rubbery bio-optoelectronic stimulator (RBOES). This ultrathin, stretchable, and self-adhesive device integrates seamlessly with the dynamic movement of cardiac tissue. Using advanced nanotechnology, the RBOES delivers optoelectronic stimulation in a minimally invasive and adaptable manner.
Laboratory and ex vivo experiments demonstrated the device’s ability to accelerate heartbeats without requiring genetic modifications, offering a safer, untethered approach to cardiac modulation. The RBOES represents a significant step forward in the integration of materials science and biomedicine, providing a promising solution for real-time cardiac stimulation.
Bioprinted Optoelectronically Active Cardiac Tissues
The second study, “Bioprinted Optoelectronically Active Cardiac Tissues”, explores a revolutionary bioprinted cardiac tissue platform that integrates light-responsive materials to create electrically generative, wireless tissue constructs for cardiac therapy. These optoelectronically active scaffolds, embedded with micro-solar cells (μ-solar cells) within a gelatin methacryloyl (GelMA)-based ink, are seeded with cardiomyocytes to enable untethered and distributed modulation of cardiac tissues.
Key findings include:
- Enhanced Functionality: The μ-solar cells effectively synchronized and accelerated the beating of cardiomyocytes, maintaining rhythmic contractions and expressing cardiac-specific biomarkers.
- Scalable Applications: The optoelectronic scaffolds demonstrated potential for both in vitro and in vivo applications, including restoring normal heart rhythms when implanted in animal models.
- Biocompatibility and Safety: The system eliminates the need for genetic modifications, ensuring safe integration and minimizing side effects associated with conventional electrical stimulators.
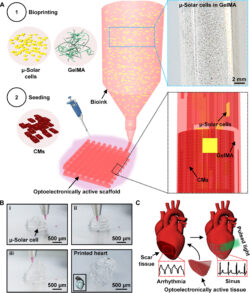 By addressing the limitations of traditional methods, such as invasive wired stimulators, this study has paved the way for noninvasive, light-controlled cardiac therapy and tissue regeneration.
By addressing the limitations of traditional methods, such as invasive wired stimulators, this study has paved the way for noninvasive, light-controlled cardiac therapy and tissue regeneration.
Together, these studies exemplify THI’s leadership in addressing critical challenges in cardiac care. By developing innovative solutions that combine bioprinting, bio-optoelectronics, and materials science, THI investigators are revolutionizing therapies for patients with heart disease.
“These projects highlight the incredible potential of bio-optoelectronics and bioprinting to redefine what is possible in cardiac tissue engineering,” said Camila Hochman-Mendez, PhD, Director of the Regenerative Medicine Research at THI. “Our work demonstrates the value of interdisciplinary collaboration in addressing complex medical challenges and showcases the forward-thinking research taking place at THI.”
“These advancements in optoelectronic cardiac stimulation represent a major leap forward in developing safer, more effective therapies for patients with heart disease,” added Abdelmotagaly Elgalad, MD, PhD., Co-Director, Cardiovascular Surgery Research, Center for Preclinical Surgical & Interventional Research. “This research is a testament to THI’s dedication to pioneering transformative solutions that merge engineering and medicine to improve patient outcomes.”
Stay tuned for more innovations from THI’s exceptional research team as they continue pushing the boundaries of cardiovascular science and engineering.




